Contoh Manual Gmp Malaysia
3 gmp implementation and management who s involved everyone lead hands are the key.
Contoh manual gmp malaysia. Packaging all packaging materials to be stored in a clean dry area on pallets or appropriate shelving. Premis perlu berdaftar dengan suruhanjaya syarikat malaysia ssm sistem haccp dan prp gmp perlu dilaksanakan sekurang kurangnya tiga 3 bulan sebelum permohonan. Gmp good manufacturing practices merupakan suatu pedoman bagi industri pangan bagaimana cara berproduksi pangan yang baik.
Open packages to. Sistem gmp perlu dibangunkan dan didokumenkan berdasarkan kepada malaysian standard 1514 latest revision. Sistem haccp perlu dibangunkan berdasarkan kepada malaysian standard 1480 latest revision.
Kuala lumpur malaysia 2008. Manual gmp perlu mengandungi dokumen minima seperti. Equipment all product handling equipment and tools to be stored off floors when not in use.
Untuk pengetahuan jumlah benar di kali 5 untuk prilaku jumlah benar di kali 10. Pengenalan tanggungjawab ini dijalankan berdasarkan. In malaysia ms 1514 2009 gmp is the requirement standards defined by ministry of health section of food safety quality division which aim to elevate the level of standards of domestic food manufacturer in producing safer food cosmetics and health supplement as well as traditional medicine.
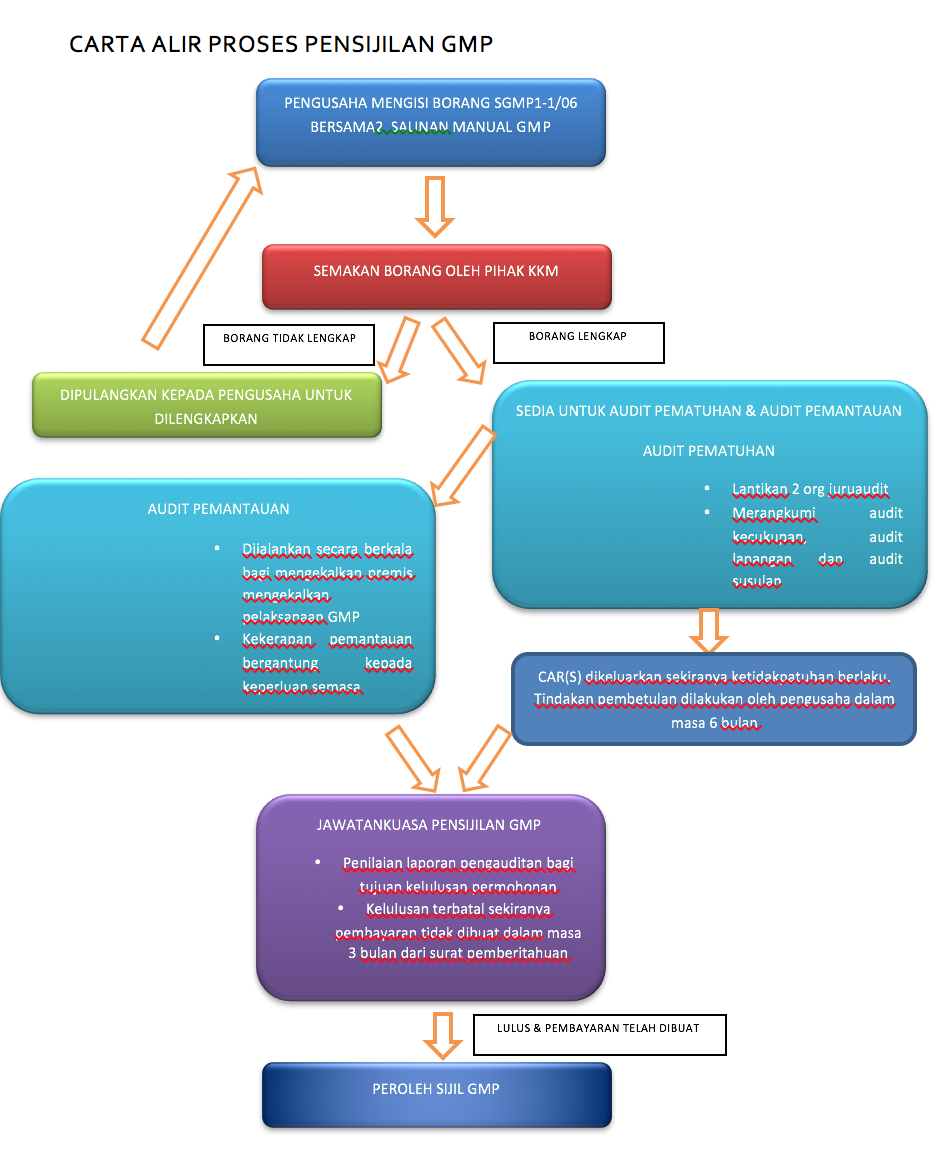
Sistem gmp perlu dilaksanakan sekurang kurangnya tiga 3 bulan sebelum permohonan. Pensijilan mesti gmp dan haccp bahagian keselamatan dan kualiti makanan kementerian kesihatan malaysia. Pengetahuan terhadap hygiene dan sanitasi perorangan termasuk pengguanaan apd perilaku hygiene dan sanitasi petugas pengolah makanan saat bekerja.
Sistem prp gmp perlu dilaksanakan. Therefore good manufacturing practice training is essential to build up your brand and increase. Gmp haccp training manual.
Contoh tabel penerapan gmp dalam penyelenggaraan makananan. Good manufacturing programs hazard analysis critical control point a system which identifies specific hazards and preventative. Premis makanan perlu mematuhi undang undang dan peraturan yang berkaitan di malaysia.
5 0 acceptable gmp evidence 5 1 one of the requirements to register an imported medicinal product in malaysia is submission of acceptable documentary evidence of the gmp compliance of the manufacturer issued by a regulatory authority. Malaysia 1st edition 2008 guidelines on good manufacturing practice for traditional medicines and health supplements introduction under the control of drugs and cosmetics regulations 1984 compliance with good manufacturing practice gmp is required as one. Kaitan gmp dengan sistem haccp dan ssop agar sistem haccp dapat berfungsi dengan baik dan efektif perlu diawali dengan.
Pengenalan kementerian kesihatan malaysia kkm bertanggungjawab melindungi orang ramai terhadap bahayanya dari segi kesihatan dan penipuan pada penyediaan penjualan dan penggunaan makanan. 2 our company is committed to the development and implementation of gmps following the principles of haccp. Gmp merupakan prasyarat utama sebelum suatu industri pangan dapat memperoleh sertifikat sistem haccp hazard analysis critical control point 1.
4 what is a gmp haccp program. Guidelines on good manufacturing practice page 2 of 87 for traditional medicines and health supplements. Jumlah responden baik.
Good manufacturing practices gmp policy manual storage chemicals all hazardous cleaning pest control and testing chemicals are to be stored away from product in designated areas.